Advanced Parenteral Closure Solutions
Pre-filled Syringe Components, Vial Components
Pharma
As the injectable pharmaceutical market shifts toward complex and sensitive drugs, Aptar Pharma has developed advanced solutions to derisk parenteral drug development.
- PremiumFill® – has enhanced specifications to comply with the EMA GMP Annex 1 revision and improve your operational efficiency.
- Ready-to-Use – our gamma sterilization process guarantees sterility at the time of use and helps reduce your operational costs.
- Rapid Transfer Port bags – essential element for aseptic filling processes that comply with Annex 1 and minimize the risk of contamination of the injectable drug product on fill-finish lines.


Unlock the Potential of your Injectable Primary Packaging
Advanced parenteral closure solutions to comply with a changing market
The pharmaceutical industry is evolving towards more complex and sophisticated drugs such as biologics and next-generation vaccines. These innovations offer new possibilities for treating and preventing various diseases but involve intricate manufacturing processes and substantial research and development investments.
Alongside this progression, regulatory expectations are also changing, with agencies imposing stricter standards for safety, efficacy, and quality.
Leveraging decades of primary packaging expertise, Aptar Pharma can help our pharmaceutical partners navigate these increasingly complex landscapes. We have developed technologies and processes that can be combined to help you bring safer and more effective therapeutic to patients, reduce the overall risk, while improving your operation and accelerating time-to-market.

- PremiumFill®
- Ready-to-Use Gamma Sterilized Solutions
- Rapid Transfer Port Bags
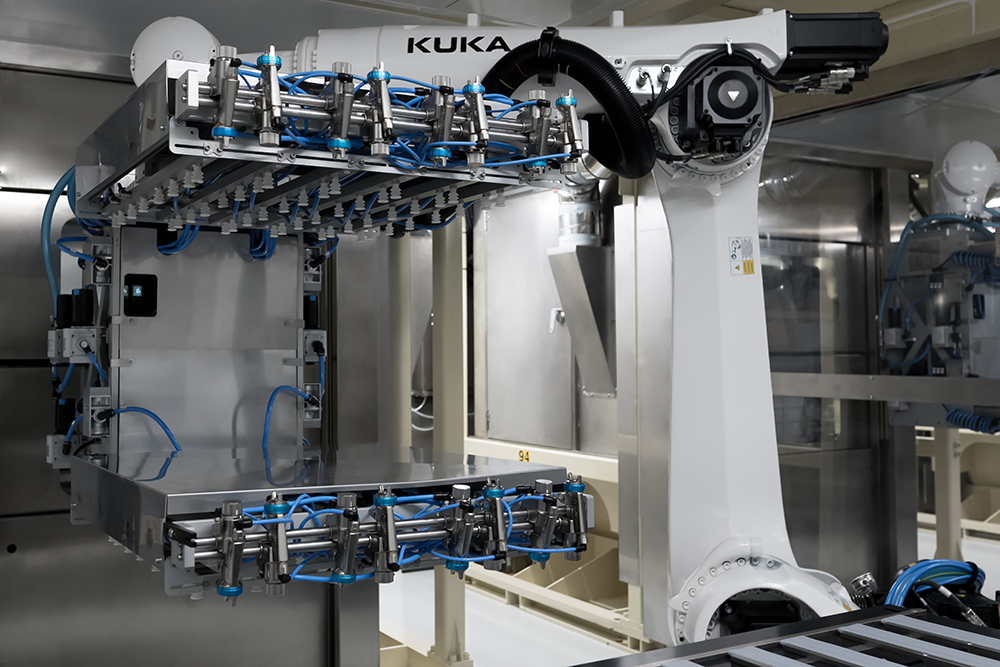
Improved specification for reduced contamination and improved operational efficiency
Our PremiumFill® vial stoppers and syringe plungers are processed in ISO-classified clean rooms from the molding all the way to final packaging, with state-of-the-art robotization. PremiumFill® enhanced particulate specification can help optimize pharmaceutical operations, reduce the risk of contamination, reduce scrap rates and meets the requirements of the recent EMA GMP Annex 1 revision.

Guaranteed sterility at the time of use to ensure patient safety & reduce your operational costs
Our formulations for vial stoppers and pre-filled syringe have been validated with gamma sterilization. Aptar Pharma’s process guarantee sterility at the point of use and help you comply to Annex 1 revisions regarding assurance of sterility. Optimize your operation and your costs by letting us guarantee the sterility of your components.

Reduce the risk of external contamination on drug product fill-finish lines
Our Ready-To-Sterilize (RTS) and Ready-To-Use (RTU) vial stoppers and syringe plungers can be supplied in a wide variety of Rapid Transfer Port bags. Connect your Rapid Transport Port bags directly to your isolator/RABS in order to prevent accidental contamination of your drug product with our aseptic fill-finish operations. Using isolators and port bags help you comply with the recent revision of Annex 1.
A premium approach to ensuring drug integrity and patient safety
When the primary packaging is in direct contact with the drug product, its cleanliness is of prime importance. Contamination, whether they are fibers, particles or micro-organism can pose serious threat to patient’s safety. Pharmaceutical manufacturers must ensure that their operations are under the strictest control from the earliest stages of their supply chains to the packaging of their finished product and the final delivery to the patient.
Aptar Pharma’s advanced solutions have been developed to reduce the risk of contamination and to ensure that each dose can be delivered safely to your patients.
- Improved packaging cleanliness with PremiumFill® with improved specification on key contamination criteria (fibers, particulate, biological).
- Sterility guaranteed at the time of use with Ready-To-Use vial stoppers and pre-filled syringe plungers.
- Prevent the accidental contamination of your drug product fill-finish operations by choosing Rapid Transfer Port bags.

Accelerate time to market and regulatory compliance
With the sky-rocketing costs of drug developments and fierce competition, any delay in bringing a drug to market can lead to significant losses. Pharmaceutical manufacturers are therefore looking to secure their drug development as quickly as possible and accelerate their time to market.
Particulate contamination and sterility issues in pharmaceuticals can be the cause of adverse effects and are among the main cause for regulatory recalls. The revision of the European Medicines Agency (EMA) Good Manufacturing Practice (GMP) Annex 1 illustrates the increased focus on these topics and now require pharmaceutical manufacturers to implement a Pharmaceutical Quality System and demonstrate they have developed a comprehensive Contamination Control Strategy (CCS) that covers their operations from their suppliers to their final product.
Aptar Pharma’s advanced solutions can be combined to help customer address these stringent requirements by offering:
- Tighter and improved specification on key contamination criteria (fibers, particulate, biological) with PremiumFill® with industrial means in place to help support your CCS.
- Meeting the sterility assurance requirements with our Ready-To-Use (RTU) gamma sterilized products and limit the risk of Tyvek-related fiber contamination.
- Compliance with Annex 1 requirements regarding the use of isolators/RABS, limits the risk of accidental contamination to support your CCS by using Rapid Transfer Port bags (RTP).

Improved operational efficiency
With the increasing cost of drugs being developed and produced, any operational inefficiency, product reject or eventual product recall can lead to significant losses for pharmaceutical companies. Selecting the right packaging component can therefore help contain these risks, ultimately mitigating the total cost of ownership for higher quality packaging and promoting long term-success for your drug on the market.
- With their improved specifications on particle contamination and overall products, PremiumFill® vial stoppers and pre-filled syringe plungers can help limit rejection rates on drug product fill-finish lines, reduce scrap rates and the overall risk of recall.
- Ready-to-Use solutions guarantee sterility at the time of use and exempts drug manufacturer to acquire sterilization equipment and validate their own process, saving space, time and costs.
- Limit the risk of accidentally introducing particles on your filling line to limit risks of reject and product recall.

PremiumFill®: a process to reach high quality standards
We developed an improved process to deliver you improved specifications on key criteria (Particle Count Index, Fibers, embedded, loose and metallic particles, stains, biological contamination). This is achieved by implementing advanced robotization, operating in ISO classified clean rooms from molding to final packaging and leveraging Aptar Pharma’s Ultraclean6 finishing process.
Ready-to-Use gamma: guaranteed sterility and demonstrated performance
Aptar Pharma’s formulations were developed, tested, validated and our sterilized products maintain their functional and chemical performances after gamma sterilization. By using PE secondary packaging only, our Ready-To-Use solutions prevent the introduction of Tyvek particles on your filling line.
Rapid Transfer Port bag: for aseptic drug-product fill-finish operations
All products, whether delivered Ready-To-Sterilize or Ready-To-Use Gamma sterilized can be packaged in a variety of Rapid Transfer Port (RTP) bags in order to meet the requirements of your specific drug product fill-finish operations. By helping to prevent the accidental introduction of particles into your aseptic filling operations, RTP bags support compliance to the recent Annex 1 revisions.
Product Details
Contact Aptar Pharma’s parenteral expert team today
Learn more about how we can support your compliance to Annex 1 revision and optimize your operations with our advanced injectable packaging solutions.
This Might Also Be of Interest

Advanced Parenteral Closure Solutions: The Perfect Fit Towards Compliance with EMA GM...
Webinars, Pharmaceutical, Innovation & Insights, Market Insights, Product Solutions

Raising the Bar for Injectable Drugs: EU GMP Annex 1 Primary Packaging Considerations
Webinars, Pharmaceutical, Market Insights, Product Solutions, Innovation & Insights, Device Innovations
Exploring Extractable and Leachable Testing Strategies for Parenterals
Publications, Pharmaceutical, Innovation & Insights, Market Insights, Product Solutions
Elevating Medication Adherence and Clinical Outcomes with Connected Drug Delivery
Publications, Pharmaceutical, Market Insights, Product Solutions, Device Innovations, Brand Differentiation
We Offer World-Leading Support Services for You at Every Stage of Your Product Development
Explore How We Serve Your Market
Request More Information
Requesting information on Advanced Parenteral Closure Solutions.
How Can We Help?


